Describe Surface Tension From Molecular Theory Using Well Labeled Diagram
Surface tension is a property of a liquid that allows them to resist external forces. The force is perpendicular to the line and tangential to the liquid surface.
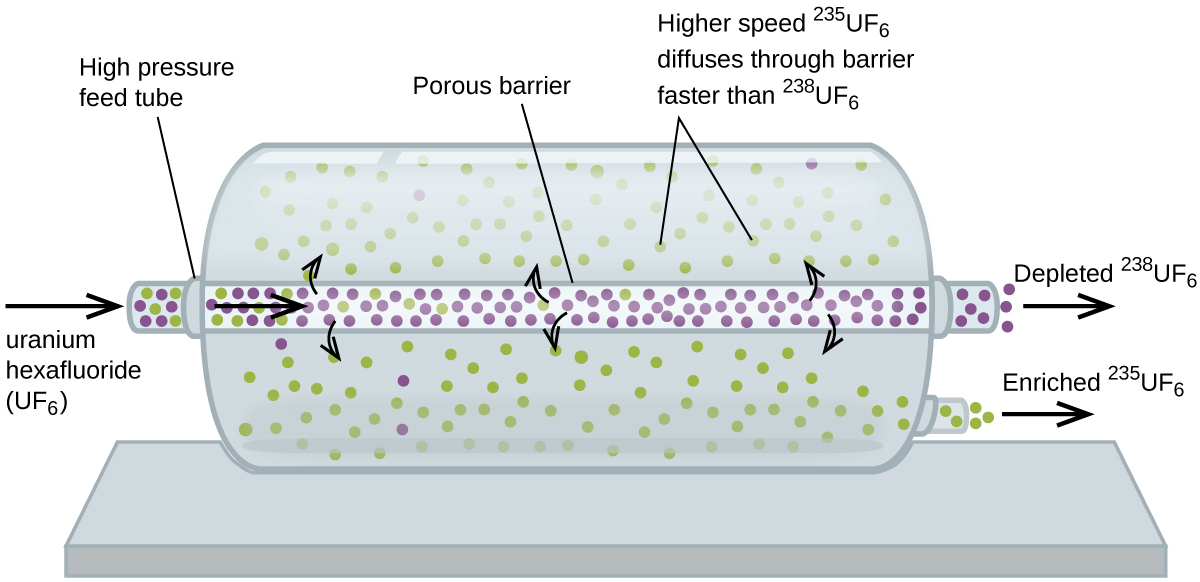
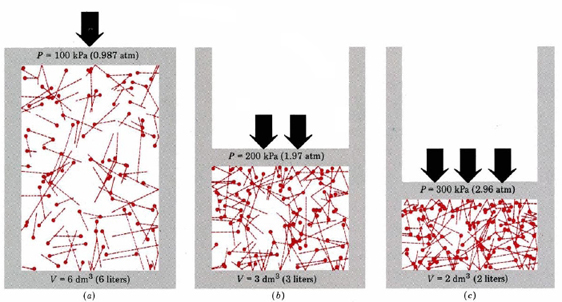
Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
Water has a surface tension of 007275 joule per square metre at 20 C 68 F.
. Relation between the Surface Energy and the Heat of Vaporization. Surface tension is responsible for the curvature of the surfaces of air and liquids. Every one of the water molecules in the bulk liquid exerts a small amount of attractive force upon its nearest neighbors.
Google Scholar Kurata M. A force that tends to pull adjacent parts of a liquids surface together thereby decreasing surface area to the smallest possible size. Surface tension is caused by a strong attraction.
The Influence of Differences in Molecular Size on the Surface Tension of Solutions. Prigogine I and J. It is what causes polar liquid solvents like water to form beads on a non-porous surface ran than a thin uniform.
Surface tension is responsible for the ability of some solid objects to float on the surface of a liquid. Surface tension denoted with the Greek variable gamma is defined as the ratio of the surface force F to the length d along which the force acts. Surface tension is a property found in many liquids at the air-liquid interface.
Surface tension is defined as the force per unit length acting perpendicular on an imaginary line drawn on the liquid surface. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. The liquid molecules which are at the surface are attracted by other molecules in downward direction.
Physics Assignment Help Explain the molecular theory of surface tension i On the average particles are separated by a distance of the order of 10-10 m and exert a force of attraction of. A molecular theory of surface tension is developed for a liquidgas interface of a one component system. Calculation of the Surface Energy.
Here the water molecules in the droplet are held. Surface tension may be expressed therefore in units of energy joules per unit area square metres. Values of the surface tension and surface excess internal energy of a number of simple liquids are obtained by numerical integration of the Kirkwood-Buff-Fowler.
Viva-Voce Surface Tension Q1. If F is the force acting on the length l of the line AB then surface tension is given by TFl. The Helmholtz free energy the quantity minimized in the van der Waals approach is.
Surface tension is a fundamental property of the surface of liquid. The property of the surface of a liquid that allows it to resist an external force due to the cohesive nature of its molecules SourcesUsage. Thermodynamics of Surface Tension.
Statistical-Mechanical Treatment of the Surface. The total energy of the ensemble of water. The attraction of the.
The tangential cohesive force acting along the unit length of the surface of a liquid T F L Where F total force along a line. As we know surface tension is given by the formula Surface tension FL. We know that F ma substituting the value in the equation we get maL.
Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to liquid based on the nature of the intermolecular. Watch Previous 2 Videos. The liquid molecules which are below the surface are in equilibrium.
7 122 195 2. It combines the concepts of cohesion and adhesion. Video Lecture Laplaces Molecular Theory of Surface Tension from Properties of Liquids chapter of Basic Physics for MSBTE Semester 1.
Surface tension is responsible for the. Gamma F d Units of Surface.
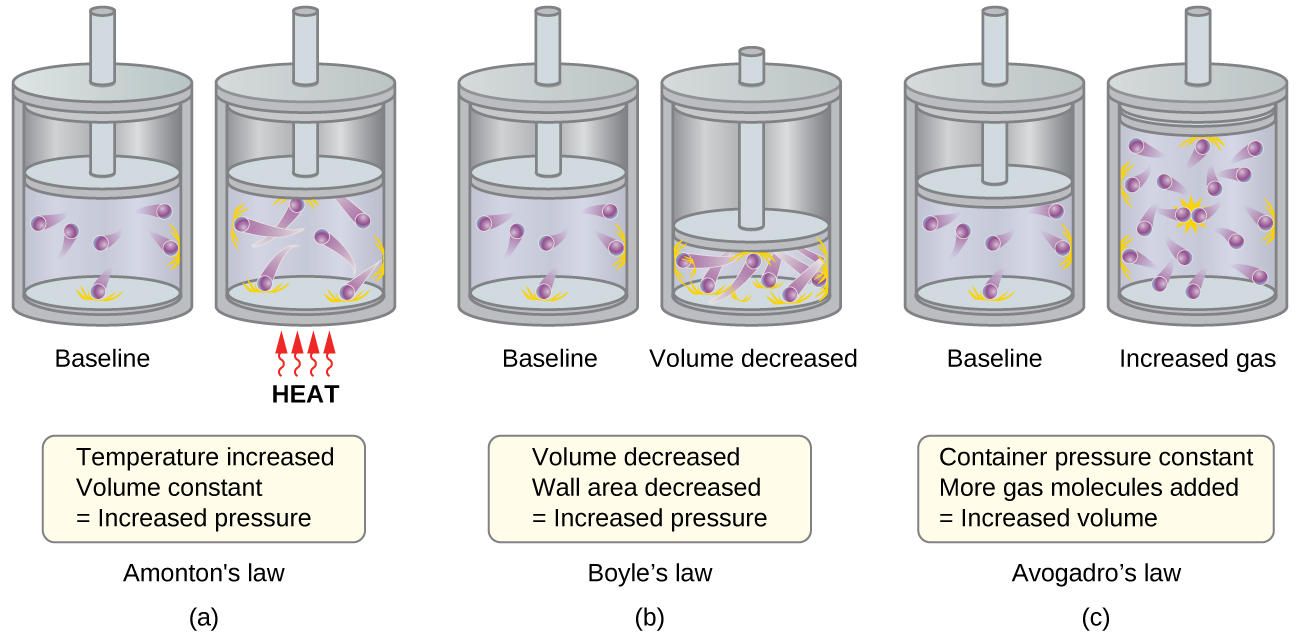
Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
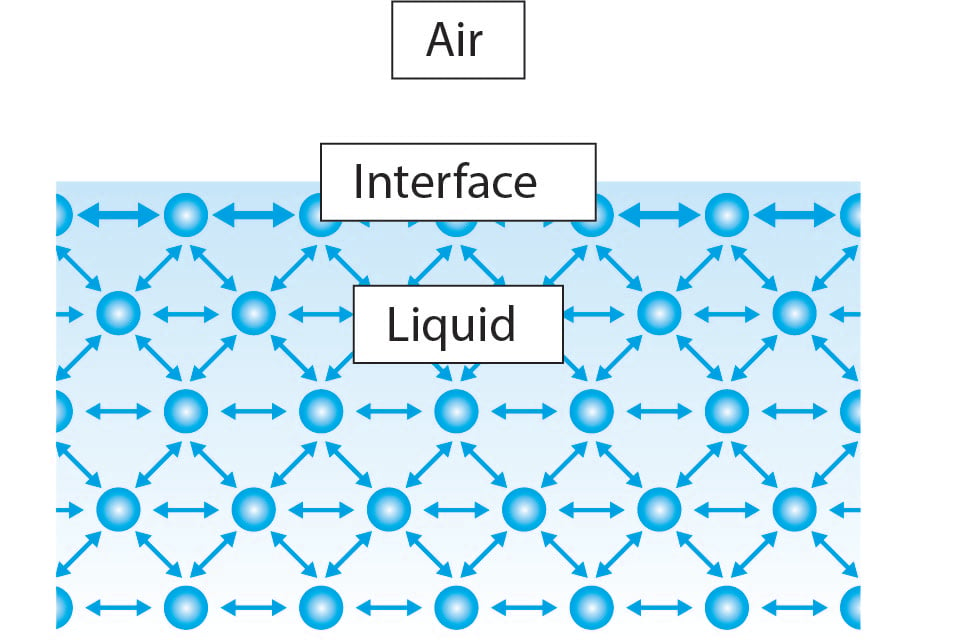
Glossary Knowledge Biolin Scientific

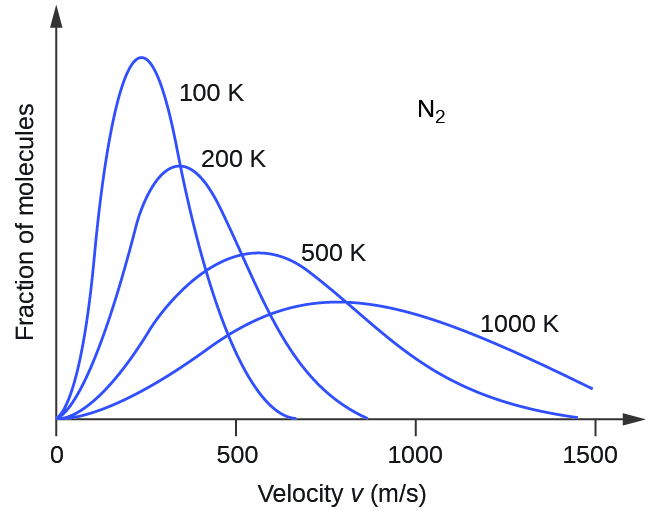
Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
Comments
Post a Comment